A US drug company says it has created the first therapy that could slow Alzheimer’s disease, and it is now ready to bring it to market.
Currently, there are no drugs that can do this – existing ones only help with symptoms.
The treatment offers new hope to patients in the early stages of the most common form of dementia,
The company BIOGEN is now seeking regulatory approval for the drug in the US after new analysis of trial data revealed some patients experienced benefits in memory, orientation, language and everyday living skills such as shopping and doing laundry.
Approval processes could take a year or two. If successful, the company aims to initially offer the drug to patients previously enrolled in clinical studies of the drug.
The announcement is somewhat surprising because the company had discontinued work on the drug in March 2019, after disappointing trial results.
The decision to seek regulatory approval of aducanumab comes after clinical studies ended in March this year.
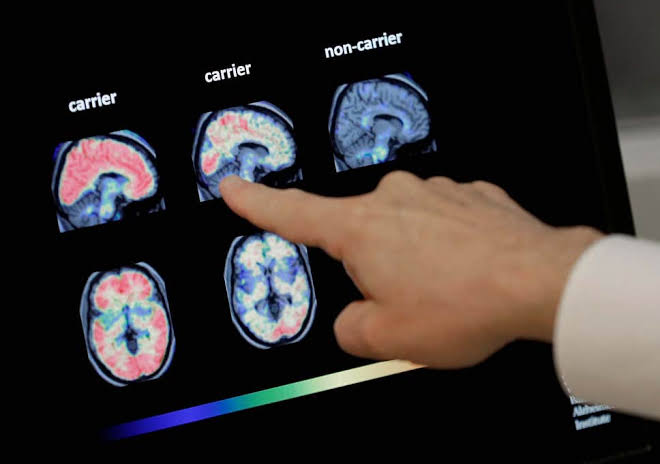
Aducanumab targets a protein called amyloid that forms abnormal deposits the brains of people with Alzheimer’s. Scientists think these plaques are toxic to brain cells and that clearing them using drugs would be a massive advance in dementia treatment, although not a cure.
Michel Vounatsos, chief executive at Biogen, said: “With such a devastating disease that affects tens of millions worldwide, today’s announcement is truly heartening in the fight against Alzheimer’s.
Dementia causes an ongoing decline in brain function, which can affect memory, thinking speed, speech, mood and movement.